September 27, 2022 Tags: Plastic Surgery News
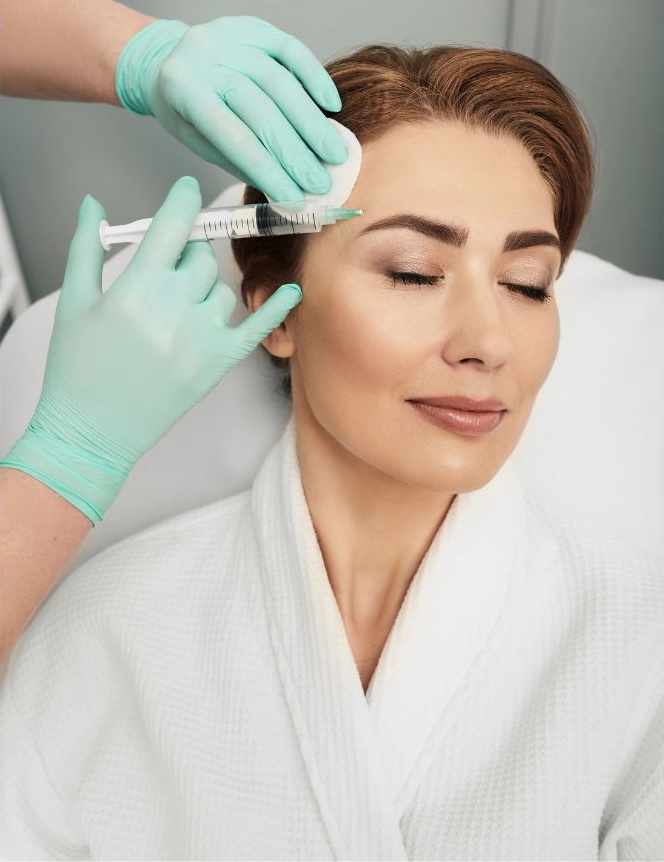
On September 8th, 2022, Revance Therapeutics, Inc., a biotechnology company focused on innovative aesthetic and therapeutic offerings, announced that the U.S. Food and Drug Administration (FDA) has approved DAXXIFY™ (DaxibotulinumtoxinA-lanm) for injection for the temporary improvement of moderate to severe frown lines, also known as glabellar lines, in adults.
DAXXIFY™ is the first and only neuromodulator stabilized with Peptide Exchange Technology™ (PXT) and is free of both human serum albumin and animal-based components. DAXXIFY™ is also able to address the duration of treatment effect, which Revance stated they believe is the greatest unmet need with existing neuromodulators for both consumers and injectors.
The U.S. approval of DAXXIFY™ was based on the data generated in the SAKURA Phase 3 clinical trial program, which included more than 2,700 patients and approximately 4,200 treatments. During this clinical trial, some patients maintained treatment results at nine months, with a six-month median duration. Results were seen as early as one day after treatment and typically seen within two days. In addition, the clinical trial found that:
74% of subjects achieved a > two-grade improvement in glabellar lines at week four per both investigator and patient assessment
88% achieved > two-grade improvement at week four per investigator assessment
98% of subjects achieved none or mild wrinkle severity at week four per investigator assessment
DAXXIFY™ is generally safe and well tolerated with no serious treatment-related adverse events reported in the clinical trials and has a safety profile consistent with other currently available neuromodulators in the aesthetics market.